/home/grassnews/public_html/wp-content/themes/zox-news/parts/post-single.php on line 153
">
Warning: Undefined array key 0 in /home/grassnews/public_html/wp-content/themes/zox-news/parts/post-single.php on line 153
Warning: Attempt to read property "cat_name" on null in /home/grassnews/public_html/wp-content/themes/zox-news/parts/post-single.php on line 153
Ferrari Selects YASA Electric Motor for SF90 Stradale, the Company’s First Hybrid Production Series Supercar
 Reading Time: 1 minute
Reading Time: 1 minute
OXFORD, England–(BUSINESS WIRE)–YASA, the world’s leading manufacturer of axial-flux electric motors,
today announced that a YASA electric motor powers Ferrari’s first hybrid
series production sports car, the SF90 Stradale. YASA have been working
closely with Ferrari over the past two and a half years, developing a
custom version of its electric motor that meets Ferrari’s demanding
performance specifications. The SF90 Stradale was launched by Ferrari on
29 May in Maranello, Italy.
YASA’s innovative axial-flux electric motor designs offer best-in-class
power and torque densities. They are ideally suited to both hybrid and
pure electric vehicle applications. The custom YASA motor in the SF90
Stradale is highly compact with a world-leading power density of
14kW/kg, allowing Ferrari to deliver unparalleled vehicle performance
whilst reducing vehicle weight. The motors will be manufactured in
YASA’s production facility in Oxford, UK.
Chris Harris, YASA’s CEO said, “We’re delighted to announce Ferrari as a
YASA customer and for Ferrari’s first ever hybrid series production car
– the SF90 Stradale – to be powered by a YASA electric motor. We’ve
worked closely with Ferrari over the past two and a half years to
develop a custom solution that meets their unique performance
requirements, aided by the fact that our companies both share the same
passion for innovation and the same unwavering commitment to excellence.
Through this long-term collaboration, we are helping to set the bar for
high-performance electric driving experiences.”
EDITOR’S NOTES
YASA http://www.yasa.com/
YASA is the world’s leading manufacturer of axial-flux electric motors
for automotive and aerospace applications. YASA’s proprietary electric
motor products offer the smallest, lightest form factor for a given
power and torque requirement. The privately-held company is based in
Oxford, the UK’s automotive manufacturing heartland. Investors include
Parkwalk Advisors and Universal Partners.
Contacts
Tamara Sword
media@yasa.com
+44
(0)7906 638351

Warning: Undefined array key 0 in /home/grassnews/public_html/wp-content/themes/zox-news/parts/post-single.php on line 493
Warning: Attempt to read property "cat_ID" on null in /home/grassnews/public_html/wp-content/themes/zox-news/parts/post-single.php on line 493
Innocan
Innocan Pharma Submits Investigational New Animal Drug Application to FDA’s Veterinary Center

HERZLIYA, Israel and CALGARY, AB, July 26, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB: INNPF) (“Innocan” or the “Company”), a pioneer in the pharmaceutical and biotechnology industries, is pleased to announce that the FDA’s Center for Veterinary Medicine (CVM) has granted the Company a sponsor fee waiver and assigned an Investigational New Animal Drug (INAD) number for its LPT-CBD (Liposome Platform Technology-Cannabidiol) product. This represents a significant step for the Company, as an INAD designation facilitates correspondence and data exchange with CVM to support LPT-CBD development as a new veterinary drug.
The Company further announced that following the assessment of LPT-CBD’s scientific package, the CVM recognized Innocan’s contribution to pursuing innovative animal drug products and technology and granted the company a sponsor fee waiver for fiscal year 2024.
Innocan’s LPT-CBD is a proprietary drug delivery platform designed to provide prolonged-release CBD for chronic pain and well-being management in animals. Over the past year, repeated administration of LPT-CBD in dogs and other animals has demonstrated both efficacy and tolerability, providing sufficient evidence for the INAD application.
“We are thrilled by CVM’s response,” said Prof. Chezy Barenholz, CSO of Innocan Pharma. “The granted INAD will allow us to advance the investigational studies of LPT-CBD and share knowledge to support future discussions with CVM on LPT-CBD’s development plan. Moreover, the fee waiver, granted by CVM, supports our development and pursuit of innovative animal drug products and technology, further validating our approach and potential impact in veterinary medicine.”
Dr. Eyal Kalo, R&D Director at Innocan, added, “LPT-CBD is a unique technology that has proven itself worthy of the INAD fee waiver granted by CVM. This will streamline our efforts to deliver a unique solution for chronic pain management to the animal market.”
About Innocan Pharma:
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD-loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
Contact Information:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+1 5162104025
+972-54-3012842
+442037699377
info@innocanpharma.com
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution Regarding Forward-Looking Information
Certain information set forth in this news release, including, without limitation, the Company’s plans for human trials of its LPT-CBD platform, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. . The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties that could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: global and local (national) economic, political, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and potential disruption of relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import/export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner). The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release. A comprehensive discussion of other risks that impact Innocan can be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedarplus.ca.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward-looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo: https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-submits-investigational-new-animal-drug-application-to-fdas-veterinary-center-302207435.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-submits-investigational-new-animal-drug-application-to-fdas-veterinary-center-302207435.html

Cannabis
Verano Announces the Opening of Zen Leaf Fairless Hills, the Company’s Newest Affiliated Dispensary in Pennsylvania, in Prime New Location
- Zen Leaf Fairless Hills, the Company’s newest affiliated dispensary in Pennsylvania, relocated from its former home in Chester to 203 Lincoln Highway, a busy thoroughfare with daily traffic of over 17,000 vehicles per day1
- As the first medical cannabis dispensary in the city, Zen Leaf Fairless Hills will offer an elevated experience for area patients, including increased convenience and accessibility with numerous point-of-sale stations and kiosks for seamless in-store browsing and ordering
- Verano’s active operations span 13 states, comprised of 142 dispensaries and 13 cultivation and processing facilities with more than 1 million square feet of cultivation capacity
CHICAGO, July 26, 2024 (GLOBE NEWSWIRE) — Verano Holdings Corp. (Cboe CA: VRNO) (OTCQX: VRNOF) (“Verano” or the “Company”), a leading multi-state cannabis company, today announced the opening of Zen Leaf Fairless Hills in Pennsylvania on Friday, July 26th, following a ceremonial ribbon cutting at 11 a.m. local time. Zen Leaf Fairless Hills is located at 203 Lincoln Highway and will be open Monday through Saturday from 9 a.m. to 8 p.m. and Sunday from 10 a.m. to 6 p.m. local time.
The dispensary is located in Bucks County, the fourth largest county in the Commonwealth with a total population of over 630,0002 residents. To increase accessibility and convenience, Zen Leaf Fairless Hills features large in-store kiosks and numerous point-of-sale stations to enhance the browsing and ordering experience for patients. To celebrate the grand opening of Zen Leaf Fairless Hills and following a ceremonial ribbon cutting, patients will be greeted with complimentary deals and doorbusters on featured branded products.
“We are excited to bring the Zen Leaf experience to local patients in Fairless Hills, where our talented team members will continue to deliver hospitality-driven care and top-quality products for local patients,” said George Archos, Verano Founder and Chief Executive Officer. “As the Pennsylvania medical cannabis patient population continues to grow, we are grateful for the opportunity to deepen our roots in Bucks County at our newest Zen Leaf location in the Commonwealth, and look forward to providing a warm and welcoming environment for current and future patients.”
Zen Leaf Fairless Hills adds another convenient outlet for Philadelphia area patients, and solidifies Verano’s footprint in the state as one of the Company’s 18 affiliated Pennsylvania dispensaries. Verano’s Pennsylvania operations also include a state-of-the-art 62,000 square foot cultivation and processing facility in Chester, where the Company produces its signature Verano Reserve flower and Troches, concentrates and vapes; (the) Essence and Savvy flower and extracts; and Avexia RSO cannabis oil and topicals. For additional convenience and accessibility, patients can choose to order ahead at ZenLeafDispensaries.com for express in-store pickup.
About Verano
Verano Holdings Corp. (Cboe CA: VRNO) (OTCQX: VRNOF), one of the U.S. cannabis industry’s leading companies based on historical revenue, geographic scope and brand performance, is a vertically integrated, multi-state operator embracing a mission of saying Yes to plant progress and the bold exploration of cannabis. Verano provides a superior cannabis shopping experience in medical and adult use markets under the Zen Leaf™ and MÜV™ dispensary banners, including Cabbage Club™, an innovative annual membership program offering exclusive benefits for cannabis consumers. Verano produces a comprehensive suite of high-quality, regulated cannabis products sold under its diverse portfolio of trusted consumer brands including Verano™, (the) Essence™, MÜV™, Savvy™, BITS™, Encore™, and Avexia™. Verano’s active operations span 13 U.S. states, comprised of 13 production facilities with over 1,000,000 square feet of cultivation capacity. Learn more at Verano.com.
Contacts:
Media
Verano
Steve Mazeika
VP, Communications
Steve.Mazeika@verano.com
Investors
Verano
Julianna Paterra, CFA
VP, Investor Relations
Julianna.Paterra@verano.com
Forward Looking Statements
This press release contains “forward-looking statements” within the meaning of the safe harbor provisions of the United States Private Securities Litigation Reform Act of 1995. Such forward-looking statements are not representative of historical facts or information or current condition, but instead represent only the Company’s beliefs regarding future events, plans, strategies, or objectives, many of which, by their nature, are inherently uncertain and outside of the Company’s control. Generally, such forward-looking statements can be identified by the use of forward-looking terminology such as “plans”, “expects” or “does not expect”, “is expected”, “budget”, “future”, “scheduled”, “estimates”, “forecasts”, “projects,” “intends”, “anticipates” or “does not anticipate”, or “believes”, or variations of such words and phrases, or may contain statements that certain actions, events or results “may”, “could”, “would”, “might” or “will be taken”, “will continue”, “will occur” or “will be achieved”. Forward-looking statements involve and are subject to assumptions and known and unknown risks, uncertainties, and other factors which may cause actual events, results, performance, or achievements of the Company to be materially different from future events, results, performance, and achievements expressed or implied by forward-looking statements herein, including, without limitation, the risk factors described in the Company’s annual report on Form 10-K for the year ended December 31, 2023, its quarterly report on Form 10-Q for the quarter ended March 31, 2024 and any subsequent quarterly reports on Form 10-Q, in each case, filed with the U.S. Securities and Exchange Commission at www.sec.gov. The Company makes no assurances and cannot predict the outcome of all or any part of the on-going litigation with Goodness Growth referenced in this press release, including whether the Company will prevail on its Notice of Application and its counterclaim, or whether Goodness Growth will prevail on its claim for damages against the Company. The forward-looking statements contained in this press release are made as of the date of this press release, and the Company does not undertake to update any forward-looking information or forward-looking statements that are contained or referenced herein, except as may be required in accordance with applicable securities laws. All subsequent written and oral forward-looking information and statements attributable to the Company or persons acting on its behalf is expressly qualified in its entirety by this notice regarding forward-looking information and statements.
###
1 Pennsylvania Department of Transportation
2 United States Census Bureau

Cannabis
Unlocking New Horizons in Health: TNR, The Niche Research Reveals the Transformative Power of Minor Cannabinoids
Wilmington, Delaware, July 25, 2024 (GLOBE NEWSWIRE) — Minor cannabinoids refer to the lesser-known compounds found in the cannabis plant, distinct from the well-known THC (tetrahydrocannabinol) and CBD (cannabidiol). While THC and CBD dominate the market, minor cannabinoids such as CBG (cannabigerol), CBC (cannabichromene), and CBN (cannabinol) are gaining attention for their potential therapeutic benefits. These compounds are extracted from both marijuana and hemp plants, with varying legal restrictions depending on their THC content. The minor cannabinoids market is poised for significant growth, driven by increasing consumer awareness and demand for alternative health and wellness products. As regulatory environments around cannabis products evolve, companies are exploring the potential of minor cannabinoids in various applications, including pharmaceuticals, nutraceuticals, cosmetics, and food and beverages.
Minor cannabinoids are being researched for their potential therapeutic effects, including anti-inflammatory, analgesic, and neuroprotective properties. This versatility facilitates product diversification in various industries. Companies are investing in research and development to create novel formulations and delivery methods for minor cannabinoids. This includes nano-emulsions, encapsulation technologies, and controlled-release systems to enhance bioavailability and efficacy. For example, in January 2022, CBDA + CBGA Tincture a new product was launched by Hometown Hero CBD. This 30ml tincture contains 600mg each of CBGA, CBDA, CBG, and CBD. Derived from hemp, the cannabinoids in this tincture comply with legal requirements across all 50 states in the USA. There is an increasing consumer preference for natural as well as plant-based remedies, which in turn is driving the demand for cannabinoid-infused products. This trend is particularly strong among younger demographics seeking alternatives to traditional pharmaceuticals. Evolving regulatory frameworks, particularly in regions like North America and Europe, are creating opportunities for legal market expansion. Regulatory clarity is crucial for market participants to navigate compliance and market entry.
Global Minor Cannabinoids Market: Key Datapoints
|
Market Value in 2023 |
US$ 17.8 Bn |
|
Market Value Forecast by 2034 |
US$ 42.3 Bn |
|
Growth Rate
|
8.2% |
|
Historical Data
|
2016 – 2022 |
|
Base Year
|
2023 |
|
Forecast Data
|
2024 – 2034 |
Increasing consumer interest in health and wellness products, coupled with the perceived therapeutic benefits of cannabinoids, is a major driver of market growth. Progressive cannabis legalization in various parts of the world, including the United States and parts of Europe, is expanding the addressable market for minor cannabinoids. Significant investments in research and development by pharmaceutical and biotechnology companies are accelerating product innovation and clinical trials. The market remains fragmented with opportunities for new entrants and niche players to introduce specialized products catering to specific consumer needs.
The COVID-19 pandemic initially disrupted supply chains and retail channels for minor cannabinoids products. However, the crisis also underscored the importance of health and wellness, leading to increased interest in natural remedies, including cannabinoids. As economies recover, the market is expected to rebound stronger.
The geopolitical tensions, such as the Russia-Ukraine conflict, have also affected global markets, including the minor cannabinoids sector. Fluctuating currency values, supply chain disruptions, and geopolitical uncertainty have impacted production and distribution channels. However, the long-term impact will depend on geopolitical developments and their influence on global trade and regulatory environments.
The minor cannabinoids market presents significant opportunities for growth and innovation, driven by evolving consumer preferences, regulatory advancements, and expanding research initiatives. Companies that can navigate regulatory complexities, invest in research and development, and respond to shifting consumer trends are well-positioned to capitalize on this emerging market. As the market matures, collaboration across sectors and regions will be crucial in unlocking the full potential of minor cannabinoids in various industries worldwide.
Global Minor Cannabinoids Market: Key Takeaways of the Report
- Cannabigerol (CBG) segment by product type is expected to grow at a CAGR of 6.7% in the minor cannabinoids market due to increasing research highlighting its potential therapeutic benefits, including anti-inflammatory, antimicrobial, and neuroprotective properties. As consumer awareness grows and regulatory environments become more favorable, there is heightened interest in CBG-based products for their diverse health applications, ranging from skincare to pharmaceutical formulations, driving sustained market demand and expansion.
- Pharmaceutical segment by application, leads the minor cannabinoids market with a significant revenue share of 35.8% owing to growing recognition of cannabinoids’ potential in therapeutic applications. Cannabinoids like CBD, CBG, and others show promise in treating conditions such as epilepsy, chronic pain, and anxiety disorders, backed by increasing clinical research and favorable regulatory developments. Pharmaceutical companies are investing heavily in cannabinoid-based drug development, driving market growth as they seek to capitalize on these compounds’ efficacy and market potential in addressing unmet medical needs.
- In 2023, Latin America is anticipated as fastest growing region in the global minor cannabinoids market due to evolving regulatory landscapes favoring cannabis legalization and cultivation. This shift is fostering a burgeoning industry infrastructure for cannabis extraction and product development. Additionally, increasing consumer acceptance of cannabinoid-based products for medicinal and wellness purposes is driving market expansion. With a vast potential consumer base and supportive regulatory frameworks, Latin America presents significant growth opportunities for companies seeking to enter or expand within the minor cannabinoids market.
Key Development:
- In December 2023, Rare Cannabinoid Company introduced Uplift Gummies infused with THC and THCV. These gummies combine the relaxing properties of Delta-9-THC with the energizing and appetite-controlling effects of CBD and THCV.
- In October 2022, High Tide Inc., a cannabis retailer, announced that its Colorado-based subsidiary, NuLeaf Naturals, had launched plant-based softgels and full-spectrum multicannabinoid oil in Manitoba. The products feature CBC, CBD, CBG, Delta-9 tetrahydrocannabinol (Delta 9), and CBN.
Browse Related Category Reports
Global Minor Cannabinoids Market:
- Aurora Europe GmbH
- BulKanna
- CBD. INC.
- Fresh Bros Hemp Company
- GCM Holdings, LLC (Global Cannabinoids)
- GenCanna.
- High Purity Natural Products.
- Laurelcrest
- Mile High Labs
- PBG Global
- Rhizo Sciences
- ZERO POINT EXTRACTION, LLC
- Other Industry Participants
Global Minor Cannabinoids Market
By Product Type
- Cannabigerol (CBG)
- Cannabichromene (CBC)
- Cannabinol (CBN)
- Cannabidivarin (CBDV)
- Tetrahydrocannabutol (THCB)
- Tetrahydrocannabivarin (THCV)
- Tetrahydrocannabiphorol (THCP)
- Others
By Application
- Pharmaceutical
- Pain Management
- Mental Health
- Sleep Disorders
- Anti-inflammatory
- Others
- Nutraceuticals
- Cosmetics and Personal Care
- Food and Beverages
- Others
By Region
- North America (U.S., Canada, Mexico, Rest of North America)
- Europe (France, The UK, Spain, Germany, Italy, Nordic Countries (Denmark, Finland, Iceland, Sweden, Norway), Benelux Union (Belgium, The Netherlands, Luxembourg), Rest of Europe)
- Asia Pacific (China, Japan, India, New Zealand, Australia, South Korea, Southeast Asia (Indonesia, Thailand, Malaysia, Singapore, Rest of Southeast Asia), Rest of Asia Pacific)
- Middle East & Africa (Saudi Arabia, UAE, Egypt, Kuwait, South Africa, Rest of Middle East & Africa)
- Latin America (Brazil, Argentina, Rest of Latin America)
Consult with Our Expert:
Jay Reynolds
The Niche Research
Japan (Toll-Free): +81 663-386-8111
South Korea (Toll-Free): +82-808- 703-126
Saudi Arabia (Toll-Free): +966 800-850-1643
United Kingdom: +44 753-710-5080
United States: +1 302-232-5106
Email: askanexpert@thenicheresearch.com
Website: www.thenicheresearch.com

-

 Cannabis2 weeks ago
Cannabis2 weeks agoIM Cannabis Shares Commence Trading on 6:1 Consolidated Basis
-

 Cannabis2 weeks ago
Cannabis2 weeks agoFractional Flow Reserve Market growing at a CAGR of 15.56% during the forecast period [2024-2030] – Exactitude Consultancy
-

 Cannabis1 week ago
Cannabis1 week agoBlank Rome Bolsters Energy Industry Team in Houston and Pittsburgh with Leading Transactional Group
-

 Cannabis1 week ago
Cannabis1 week agoManitoba Harvest Hemp Foods and Brightseed® Introduce New Coffee and Chocolate Flavors in Organic Bioactive Fiber Supplement for Gut Health
-

 Cannabis5 days ago
Cannabis5 days agoEurope Medical Cannabis Oil Market Set to Reach Valuation of USD 2,395.83 Million by 2032 | Astute Analytica
-
 Cannabis4 days ago
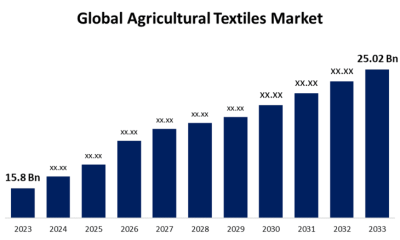
Cannabis4 days agoGlobal Agricultural Textiles Market Size To Worth USD 25.02 Billion By 2033 | CAGR of 4.70%
-

 Cannabis2 days ago
Cannabis2 days agoUnlocking New Horizons in Health: TNR, The Niche Research Reveals the Transformative Power of Minor Cannabinoids
-

 Cannabis18 hours ago
Cannabis18 hours agoVerano Announces the Opening of Zen Leaf Fairless Hills, the Company’s Newest Affiliated Dispensary in Pennsylvania, in Prime New Location














