Innocan
Innocan Pharma Unveils Groundbreaking Veterinary Breakthroughs at EAVPT 2023 Congress

HERZLIYA, Israel and CALGARY, AB, June 22, 2023 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB: INNPF), a leading innovator in pharmaceutical solutions for Human & Animal health, proudly announces its participation in the 15th International Congress organized by the Executive Committee of the European Association of Veterinary Pharmacology and Toxicology (EAVPT), in Bruges, Belgium from July 2 to July 5, 2023.
The highlight of Innocan Pharma’s participation at the EAVPT Congress will be Innocan Pharma’s presentation of two recently announced groundbreaking studies: “Pharmacokinetics and therapeutic efficacy of liposomal cannabidiol (CBD) injection: a pilot clinical study in dogs with naturally occurring osteoarthritis” and “Liposomal cannabidiol (CBD) injection: A novel therapeutic formulation is on the horizon”.
Innocan Pharma’s CEO, Iris Bincovich, expresses her excitement about this remarkable opportunity: “This is an especially thrilling moment for Innocan Pharma as we have already made substantial veterinary breakthroughs that we are eager to share. Our commitment to advancing animal health and well-being is unwavering, and EAVPT 2023 provides us with the ideal scientific forum to showcase our latest achievements.”
Innocan Pharma invites attendees to visit its booth # 16 to meet the dedicated team and learn more about its groundbreaking veterinary solutions. The Company believes its innovative products and cutting-edge research are poised to revolutionize animal health and provide new avenues for effective treatments.
EAVPT Congresses held once every three years, serve as a prestigious platform for veterinary pharmacologists, toxicologists, industry experts in animal health, government regulators, clinicians, academics, and professionals from human health and medicine to collaborate, exchange groundbreaking ideas, foster innovation, and engage in meaningful discussions.
About Innocan
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD- loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. (ii) CLX CBD-loaded exosomes platform that may hold the potential to provide a highly synergistic effect of regenerating and anti- inflammatory properties targeting the Central Nervous System (CNS). In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
For further information, please contact:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+15162104025
+972-54-3012842
+442037699377
info@innocanpharma.com
Dr. Eva Reuter
Investment Relation- Germany
+46-69-1532-5857
e.reuter@dr-reuter.eu
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution regarding forward-looking information
Certain information set forth in this news release, including, without limitation, information regarding research and development, collaborations, the filing of potential applications with the FDA and other regulatory authorities, the potential achievement of future regulatory milestones, the potential for treatment of conditions and other therapeutic effects resulting from research activities and/or the Company’s products, requisite regulatory approvals and the timing for market entry, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of requisite production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties which could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: general global and local (national) economic, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import / export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner) and availability in each market of product inputs and finished products. The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release concerning the timing of launch of product distribution. A comprehensive discussion of other risks that impact Innocan can also be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedar.com.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo: https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-unveils-groundbreaking-veterinary-breakthroughs-at-eavpt-2023-congress-301858338.html

Innocan
Innocan Pharma Submits Investigational New Animal Drug Application to FDA’s Veterinary Center

HERZLIYA, Israel and CALGARY, AB, July 26, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB: INNPF) (“Innocan” or the “Company”), a pioneer in the pharmaceutical and biotechnology industries, is pleased to announce that the FDA’s Center for Veterinary Medicine (CVM) has granted the Company a sponsor fee waiver and assigned an Investigational New Animal Drug (INAD) number for its LPT-CBD (Liposome Platform Technology-Cannabidiol) product. This represents a significant step for the Company, as an INAD designation facilitates correspondence and data exchange with CVM to support LPT-CBD development as a new veterinary drug.
The Company further announced that following the assessment of LPT-CBD’s scientific package, the CVM recognized Innocan’s contribution to pursuing innovative animal drug products and technology and granted the company a sponsor fee waiver for fiscal year 2024.
Innocan’s LPT-CBD is a proprietary drug delivery platform designed to provide prolonged-release CBD for chronic pain and well-being management in animals. Over the past year, repeated administration of LPT-CBD in dogs and other animals has demonstrated both efficacy and tolerability, providing sufficient evidence for the INAD application.
“We are thrilled by CVM’s response,” said Prof. Chezy Barenholz, CSO of Innocan Pharma. “The granted INAD will allow us to advance the investigational studies of LPT-CBD and share knowledge to support future discussions with CVM on LPT-CBD’s development plan. Moreover, the fee waiver, granted by CVM, supports our development and pursuit of innovative animal drug products and technology, further validating our approach and potential impact in veterinary medicine.”
Dr. Eyal Kalo, R&D Director at Innocan, added, “LPT-CBD is a unique technology that has proven itself worthy of the INAD fee waiver granted by CVM. This will streamline our efforts to deliver a unique solution for chronic pain management to the animal market.”
About Innocan Pharma:
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD-loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
Contact Information:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+1 5162104025
+972-54-3012842
+442037699377
info@innocanpharma.com
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution Regarding Forward-Looking Information
Certain information set forth in this news release, including, without limitation, the Company’s plans for human trials of its LPT-CBD platform, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. . The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties that could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: global and local (national) economic, political, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and potential disruption of relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import/export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner). The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release. A comprehensive discussion of other risks that impact Innocan can be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedarplus.ca.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward-looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo: https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-submits-investigational-new-animal-drug-application-to-fdas-veterinary-center-302207435.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-submits-investigational-new-animal-drug-application-to-fdas-veterinary-center-302207435.html

Innocan
Innocan Pharma Announces Successful Preliminary Safety Evaluation of LPT-CBD in Minipigs

HERZLIYA, Israel and CALGARY, AB, June 11, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB: INNPF) (“Innocan” or the “Company”), a pioneer in the pharmaceutical and biotechnology industries, is pleased to announce the success and conclusion of a preliminary safety evaluation of Innocan’s single injection and sustained-release LPT-CBD conducted on minipigs. The animals demonstrated excellent drug tolerance and did not exhibit any drug-related adverse events.
Recognized by the FDA as an excellent model for toxicology, small breeds of miniature domestic pigs known as minipigs share strong similarities with humans in crucial aspects such as drug metabolism, skin structure, genetics, and physiological mechanisms. In this preliminary safety study, minipigs received a single subcutaneous injection of LPT-CBD and were closely monitored for pharmacokinetics and basic safety parameters over one month. Encouragingly, the animals all exhibited good drug tolerance and did not manifest any drug-related adverse reactions.
“We are thrilled with these findings, which further underpin the safety profile of LPT-CBD following a single injection,” commented Dr. Eyal Kalo, the R&D Director of Innocan Pharma. “With each new data point collected for LPT-CBD, we make significant strides in our quest to revolutionize patient care through sustained-release therapy. Our efforts to continuously gather data to fully characterize LPT-CBD are paramount in our journey towards its ultimate approval.”
Professor Chezy Barenholz the CSO of Innocan Pharma added, “These results are immensely gratifying and hold significant promise as they highlight the characteristics of LPT-CBD in a physiological setting similar to humans.”
The study involved administering three ascending doses of LPT-CBD via subcutaneous injection in minipigs, followed by comprehensive monitoring of pharmacokinetics and safety parameters for 28 days. Throughout the study, the minipigs demonstrated excellent drug tolerance, as evidenced by blood clinical parameters whithin normal range, healthy appetite, and normal behavior. These findings are consistent with prior safety evaluations conducted with LPT-CBD on diverse animal models including goats and dogs, affirming the drug’s favorable tolerability profile following both single and repeated use.
Grant of Restricted Share Units
The Company has granted an aggregate of 290,000 restricted share units (each, an “RSU“) to consultants. Each RSU entitles the recipient to receive one common share of the Company (a “Common Share“) on vesting. A total of 150,000 RSUs vest on May 30, 2024, and 140,000 RSUs vest on September 30, 2024. The RSUs and the underlying Common Shares are subject to a statutory hold period of four months and one day expiring on October 1, 2024.
Innocan also announces that it granted 2,380,000 stock options to employees and consultants to the Company. These options have a strike price of $0.28, with various vesting periods up to 12 months. All options expire on May 30, 2029.
About Innocan Pharma:
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD-loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
Contact Information:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+1 5162104025
+972-54-3012842
+442037699377
info@innocanpharma.com
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution Regarding Forward-Looking Information
Certain information set forth in this news release, including, without limitation, the Company’s plans for human trials of its LPT-CBD platform, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties that could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: global and local (national) economic, political, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and potential disruption of relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import/export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner). The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release. A comprehensive discussion of other risks that impact Innocan can be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedarplus.ca.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward-looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo: https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-announces-successful-preliminary-safety-evaluation-of-lpt-cbd-in-minipigs-302169232.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-announces-successful-preliminary-safety-evaluation-of-lpt-cbd-in-minipigs-302169232.html

Innocan
Innocan Pharma Reports First Quarter 2024 Results with Revenue Growth of over 4X to $6.8 Million

HERZLIYA, Israel and CALGARY, AB, May 27, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTC: INNPF) (the “Company” or “Innocan”), a pharmaceutical technology company focusing on developing innovative drug delivery platform technologies, is pleased to announce its consolidated financial results for the three-month period ended March 31, 2024.
First Quarter 2024 Financial Highlights
- Revenues increased 334% year-over-year to US$6.8 million, compared to US$1.6 million in the first quarter of 2023, and increased 38% quarter-over-quarter, compared to US$4.9 million in the prior quarter. This significant increase in revenue was primarily due to strong sales growth of Innocan’s subsidiary, BI Sky Global Ltd.
Infographic – https://mma.prnewswire.com/media/2422871/Figure_A_Infographic.jpg
- Gross Profit increased 338% year-over-year to US$6.0 million, compared to US$1.4 million in the first quarter of 2023, and increased 40% quarter-over-quarter, compared to US$4.3 million in the prior quarter.
Management Comments
Iris Bincovich, the CEO of Innocan commented: “Our LPT-CBD technology has made strong advancements in the past few months. We very much look forward to our upcoming meeting with the FDA in July where they will review and advise on our clinical plan for bringing our LPT-CBD technology to market for chronic pain.”
Added Bincovich, “We are very excited about the potential of our LPT technology. It provides for prolonged release of CBD into the blood over extended periods, enabling the management of chronic pain over time. We have already demonstrated this successfully in several animal models, showing detectable levels of CBD in plasma for more than a month after a single injection.”
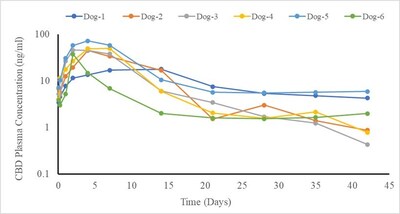
Infographic – https://mma.prnewswire.com/media/2422870/Figure_B_Infographic.jpg
Roni Kamhi, CEO of BI Sky Global, subsidiary of Innocan and COO of Innocan Pharma, commented, “We are excited with the performance of our online platform and very pleased with the 38% sequential revenue growth in the first quarter. In fact, in only one quarter, we have achieved more than half of the revenues that we recorded in the whole of 2023. Looking ahead, we anticipate continuing this growth rate by introducing new product categories and innovative formulations to our growing customer base.”
Additional information concerning Innocan’s consolidated financial statements and related management’s discussion and analysis for the three months ended March 31, 2024, can be found on the Company’s profile at www.sedar.com
About Innocan
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies based on advanced cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD- loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Pain Management and Epilepsy. (ii) CLX CBD-loaded exosomes platform that may hold the potential to provide a highly synergistic effect of regenerating and anti-inflammatory properties targeting the central nervous system. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment, Innocan has established a joint venture by the name of BI Sky Global Ltd. that focuses on advanced targeted online sales. https://innocanpharma.com/
For further information, please contact:
Iris Bincovich, CEO
+1-516-210-4025
+972-54-3012842
+442037699377
info@innocanpharma.com
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Cautionary note regarding forward-looking information
Certain information set forth in this news release, including, without limitation, information regarding research and development, collaborations, the filing of potential applications with the FDA and other regulatory authorities, the potential achievement of future regulatory milestones, the potential for treatment of conditions and other therapeutic effects resulting from research activities and/or the Company’s products, requisite regulatory approvals and the timing for market entry, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of requisite production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties which could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: general global and local (national) economic, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import / export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner) and availability in each market of product inputs and finished products. The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release concerning the timing of launch of product distribution. A comprehensive discussion of other risks that impact Innocan can also be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedar.com.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo: https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg

![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-reports-first-quarter-2024-results-with-revenue-growth-of-over-4x-to-6-8-million-302156220.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-reports-first-quarter-2024-results-with-revenue-growth-of-over-4x-to-6-8-million-302156220.html

-

 Cannabis2 weeks ago
Cannabis2 weeks agoIM Cannabis Shares Commence Trading on 6:1 Consolidated Basis
-

 Cannabis1 week ago
Cannabis1 week agoBlank Rome Bolsters Energy Industry Team in Houston and Pittsburgh with Leading Transactional Group
-

 Cannabis2 weeks ago
Cannabis2 weeks agoFractional Flow Reserve Market growing at a CAGR of 15.56% during the forecast period [2024-2030] – Exactitude Consultancy
-

 Cannabis1 week ago
Cannabis1 week agoManitoba Harvest Hemp Foods and Brightseed® Introduce New Coffee and Chocolate Flavors in Organic Bioactive Fiber Supplement for Gut Health
-

 Cannabis5 days ago
Cannabis5 days agoEurope Medical Cannabis Oil Market Set to Reach Valuation of USD 2,395.83 Million by 2032 | Astute Analytica
-
 Cannabis4 days ago
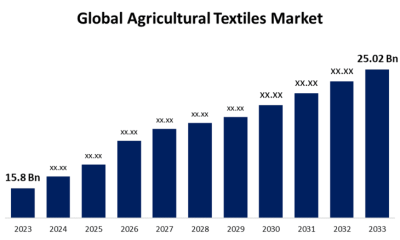
Cannabis4 days agoGlobal Agricultural Textiles Market Size To Worth USD 25.02 Billion By 2033 | CAGR of 4.70%
-

 Cannabis2 days ago
Cannabis2 days agoUnlocking New Horizons in Health: TNR, The Niche Research Reveals the Transformative Power of Minor Cannabinoids
-

 Cannabis23 hours ago
Cannabis23 hours agoVerano Announces the Opening of Zen Leaf Fairless Hills, the Company’s Newest Affiliated Dispensary in Pennsylvania, in Prime New Location



