Innocan
Innocan Pharma Announces Private Placement of Units at a 9% Prime Above Share Price

HERZLIYA, Israel, and CALGARY, Alberta, July 25, 2023 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTC: INNPF) (the “Company” or “Innocan“) is pleased to announce that it intends to complete a non-brokered private placement of up to 17,220,000 units of the Company (the “Units“) at a price of C$0.23 per Unit for gross proceeds up to C$3,960,600 (the “Offering“). The Offering is expected to close on or around July 20, 2023.
Each Unit will be comprised of: (i) one (1) common share in the capital of the Company (each a “Common Share“); (ii) one-half of one (1) Class A common share purchase warrant (each whole Class A common share purchase warrant, a “Class A Warrant“); and (iii) one-half of one (1) Class B common share purchase warrant (each whole Class B common share purchase warrant, a “Class B Warrant“).
Each Class A Warrant will entitle the holder thereof to purchase one Common Share at a price of C$0.29 for a period of three (3) years from the date of issuance. Each Class B Warrant will entitle the holder thereof to purchase one Common Share at a price of C$0.40 for a period of five (5) years from the date of issuance.
Innocan intends to use the proceeds of the Offering for working capital and general corporate purposes.
The securities issued in connection with the Offering will be subject to a hold period of four months and one day from the date of issuance, in accordance with applicable Canadian securities laws.
This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities described in this news release in the United States. Such securities have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the “U.S. Securities Act“), or any state securities laws, and, accordingly, may not be offered or sold within the United States, or to or for the account or benefit of persons in the United States or “U.S. Persons”, as such term is defined in Regulation S promulgated under the U.S. Securities Act, unless registered under the U.S. Securities Act and applicable state securities laws or pursuant to an exemption from such registration requirements.
About Innocan
Innocan is a pharmaceutical technology company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprised of cannabinoids science to treat various conditions and improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD-loaded liposome platform, which facilitates exact dosing and the prolonged and controlled release of CBD into the blood stream; the LPT delivery platform research is in the preclinical trial phase for two indications: epilepsy and pain management; and (ii) CLX CBD-loaded exosomes platform that may hold the potential to provide a highly synergistic effect of regenerating and anti- inflammatory properties targeting the Central Nervous System (CNS). In the consumer wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment, Innocan has established a joint venture by the name of BI Sky Global Ltd., which focuses on developing advanced targeted online sales.
The Company website is www.innocanpharma.com.
For further information, please contact:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+ 15162104025
+972-54-3012842
+442037699377
[email protected]
Dr. Eva Reuter
Investment Relations – Germany
+46-69-1532-5857
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPTED RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution regarding forward-looking information
Certain information set forth in this news release is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions relating to the Offering, including the terms, timing, potential completion, and the use of proceeds of the Offering.
Forward-looking information is subject to various risks and uncertainties which could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: the ability of the Company to satisfy the conditions to closing of the Offering; that the Offering may not be completed on the terms and timeline indicated, or at all; that the Company’s use of proceeds of the Offering may differ from those indicated; general global and local (national) economic, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; research and development activities; and relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import / export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner) and availability in each market of product inputs and finished products. The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release. A comprehensive discussion of other risks that impact Innocan can also be found in Innocan’s public disclosure and filings which are available under Innocan’s profile at www.sedar.com.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo – https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-announces-private-placement-of-units-at-a-9-prime-above-share-price-301884134.html

Innocan
Innocan Pharma Announces Closing of Private Placement and Grant of Stock Options

HERZLIYA, Israel and CALGARY, Alberta, Aug. 29, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB: INNPF) (“Innocan” or the “Company”), a pioneer in the pharmaceutical and biotechnology industries, is pleased to announce that it has completed its previously announced non-brokered private placement offering of 5,025,725 units of the Company (the “Units”) at a price of C$0.22 per Unit for gross proceeds of C$1,105,659.50 (the “Offering”).
Each Unit is comprised of: (i) one (1) common share in the capital of the Company (each a “Common Share”); and (ii) one (1) common share purchase warrant (each a “Warrant”). Each Warrant will entitle the holder thereof to purchase one Common Share at a price of C$0.32 for a period of four (4) years from the date of issuance.
Innocan intends to use the proceeds of the Offering for working capital and general corporate purposes.
The securities issued to Canadian subscribers in connection with the Offering are subject to a hold period of four months and one day from the date of issuance, in accordance with applicable Canadian securities laws.
Iris Bincovich, Chief Executive Officer of the Company, stated “we are very pleased with our successful offering. I would like to extend my sincere gratitude to our investors for their unwavering support. We see this as a strong vote of confidence by both existing and new investors which demonstrates investor support of our vision and strategic direction. These new funds will provide us with additional working capital to enable us to capitalize on new opportunities and allow us to advance strongly on our growth plans.”
The Company is also pleased to announce that it has granted an aggregate of 300,000 stock options (each an “Option“) to certain consultants of the Company pursuant to the Company’s stock option plan (the “Plan“). Each Option may be exercised for one (1) common share in the capital of the Company (each, a “Share“) at a price of $0.25 per Share. The Options expire on August 27, 2029.
All Options granted vest in accordance with the following vesting schedule: (i) 1/3rd of the Options vested immediately at grant; (ii) 1/3rd of the Options will vest on February 28, 2025; and (iii) 1/3rd will vest on August 27, 2025; all subject to the terms and conditions of the Plan.
About Innocan Pharma:
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD-loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
Contact Information:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+1 5162104025
+972-54-3012842
+442037699377
[email protected]
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Logo – https://mma.prnewswire.com/media/2046271/4883982/Innocan_Pharma_Corporation_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-announces-closing-of-private-placement-and-grant-of-stock-options-302234455.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-announces-closing-of-private-placement-and-grant-of-stock-options-302234455.html

Innocan
Innocan Pharma’s Subsidiary BI Sky Global Successfully Completes FDA MoCRA Registration

Innocan Pharma Announces Private Placement
HERZLIYA, Israel and CALGARY, Alberta, Aug. 22, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTCQB: INNPF) (“Innocan” or the “Company”), a pioneer in the pharmaceutical and biotechnology industries, is pleased to announce that its subsidiary, BI Sky Global (BI), successfully completed the registration of all its products under the FDA’s Modernization of Cosmetics Regulation Act of 2022 (MoCRA).
The Company further announced that following a thorough evaluation of BI’s manufacturing controls and raw materials, all its products successfully met the stringent quality standards set by the FDA, fulfilling all compliance requirements necessary for the completion of MoCRA registration.
MoCRA mandates that all facilities involved in the manufacturing and processing of cosmetic products for sale in the United States must register with the U.S. Food and Drug Administration (FDA). This enforces rigorous quality standards and ensures that only high-quality cosmetic brands can be sold in the U.S. market.
“Successful registration of our products and fully meeting the FDA’s stringent standards is a significant milestone for us, which ensures our continued retail presence in the U.S.” said Roni Kamhi, CEO of BI Sky Global. “It very much demonstrates our commitment to the highest of standards and aligns with our mission to bring top quality products to the market.”
In addition, Innocan announced that it intends to complete a non-brokered private placement of up to 4,000,000 units of the Company (the “Units”) at a price of C$0.22 per Unit for gross proceeds up to C$880,000, provided that the Company may increase the size of the private placement by up to 30% in its sole discretion to cover any over-allotments, (together, the “Offering”). The Offering is expected to close on or around August 29, 2024. Each Unit will be comprised of: (i) one (1) common share in the capital of the Company (each a “Common Share”); and (ii) one (1) common share purchase warrant (each a “Warrant”). Each Warrant will entitle the holder to purchase one Common Share at a price of C$0.32 for a period of four (4) years from the date of issuance. Innocan intends to use the proceeds of the Offering for working capital and general corporate purposes.
The securities issued to Canadian subscribers in connection with the Offering will be subject to a hold period of four months and one day from the date of issuance, in accordance with applicable Canadian securities laws. This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities described in this news release in the United States. Such securities have not been, and will not be, registered under the United States Securities Act of 1933, as amended (the “U.S. Securities Act”), or any state securities laws, and, accordingly, may not be offered or sold within the United States, or to or for the account or benefit of persons in the United States or “U.S. Persons”, as such term is defined in Regulation S promulgated under the U.S. Securities Act, unless registered under the U.S. Securities Act and applicable state securities laws or pursuant to an exemption from such registration requirements.
About Innocan Pharma:
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies comprises with cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD-loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Epilepsy and Pain Management. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment Innocan has established a Joint Venture by the name of BI Sky Global Ltd. that focuses developing on advanced targeted online sales. https://innocanpharma.com/
Contact Information:
For Innocan Pharma Corporation:
Iris Bincovich, CEO
+1 5162104025
+972-54-3012842
+442037699377
[email protected]
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Caution Regarding Forward-Looking Information
Certain information set forth in this news release, including, without limitation, the Company’s plans for human trials of its LPT-CBD platform and its intention to complete the Offering, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties that could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: global and local (national) economic, political, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and potential disruption of relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import/export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner). The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release. A comprehensive discussion of other risks that impact Innocan can be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedarplus.ca.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward-looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo – https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg
![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharmas-subsidiary-bi-sky-global-successfully-completes-fda-mocra-registration-302228789.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharmas-subsidiary-bi-sky-global-successfully-completes-fda-mocra-registration-302228789.html

Innocan
Innocan Pharma Reports Second Quarter 2024 Results with Revenue Growth of over 2.8X to $8.6 Million
Innocan Pharma Reports Half Year 2024 Results with Revenue Growth of over 3.3X to $15.4 Million
HERZLIYA, Israel and CALGARY, AB, Aug. 12, 2024 /PRNewswire/ — Innocan Pharma Corporation (CSE: INNO) (FSE: IP4) (OTC: INNPF) (the “Company” or “Innocan”), a pharmaceutical technology company focusing on developing innovative drug delivery platform technologies, is pleased to announce its consolidated financial results for the three and six-month periods ended June 30, 2024.
Second Quarter 2024 Financial Highlights
- Revenues increased 177% to US$8.6 million, compared to US$3.1 million in the second quarter of 2023, and increased 28% compared to US$6.8 million in the first quarter of 2024. This significant increase in revenue was primarily due to strong sales growth of Innocan’s subsidiary BI Sky Global Ltd.
Infographic – https://mma.prnewswire.com/media/2480462/Innocan_Figure_A_Infographic.jpg
- Gross Profit increased 201% to US$8.0 million, compared to US$2.7 million in the second quarter of 2023, and increased 33% compared to US$6.0 million in the first quarter of 2024.
- Operating Profit increased by US$0.97 million to US$0.53 million compared to an operating loss of US$0.44 million in the second quarter of 2023 and increased by US$1.7 million compared to an operating loss of US$1.2 million in the first quarter of 2024.
- Net Profit increased by US$1.35 million to US$0.95 million, compared to a net loss of US$0.4 million in the second quarter of 2023, and increased by US$2.4 million to a net loss of US$1.45 million in the first quarter of 2024.
Management Comments
Iris Bincovich, the CEO of Innocan commented: “Our LPT-CBD technology continues to make solid advancements, and we are very pleased with our progress. At the end of July, we achieved a milestone and met with the FDA to discuss our clinical plan for bringing our LPT-CBD technology to market for chronic pain. We look forward to their response in the coming weeks.”
Bincovich added, “We are becoming increasingly excited about the potential of our LPT technology, enabling the prolonged release of CBD over extended periods to manage chronic pain. In the past year, we have successfully demonstrated our technology in several animal models, showing detectable levels of CBD in plasma for more than a month after a single injection.”
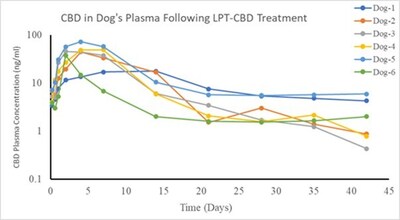
Infographic – https://mma.prnewswire.com/media/2480463/Innocan_Figure_B_Infographic.jpg
Roni Kamhi, CEO of BI Sky Global, subsidiary of Innocan, and COO of Innocan Pharma, commented, “We are very happy to share that Innocan is moving forward, achieving its milestones in both pillars Human Health and personal care.
We also see revenue growth for the last 9 quarters in our subsidiary BI SKY GLOBAL, we are very pleased with the performance of our online platform and proud of our 38% revenue growth over the prior quarter. In the first half of 2024, we have already achieved revenues exceeding our total revenue from last year. Looking ahead, we expect to continue our accelerated growth as we introduce new product categories and innovative formulations to our fast-growing customer base.”
Additional information concerning Innocan’s consolidated financial statements and related management’s discussion and analysis for the three months ended June 30, 2024, can be found on the Company’s profile at www.sedarplus.ca.
About Innocan
Innocan is a pharmaceutical tech company that operates under two main segments: Pharmaceuticals and Consumer Wellness. In the Pharmaceuticals segment, Innocan focuses on developing innovative drug delivery platform technologies based on advanced cannabinoids science, to treat various conditions to improve patients’ quality of life. This segment involves two drug delivery technologies: (i) LPT CBD- loaded liposome platform facilitating exact dosing and the prolonged and controlled release of CBD into the blood stream. The LPT delivery platform research is in the preclinical trial phase for two indications: Pain Management and Epilepsy. (ii) CLX CBD-loaded exosomes platform that may hold the potential to provide a highly synergistic effect of regenerating and anti-inflammatory properties targeting the central nervous system. In the Consumer Wellness segment, Innocan develops and markets a wide portfolio of innovative and high-performance self-care products to promote a healthier lifestyle. Under this segment, Innocan has established a joint venture by the name of BI Sky Global Ltd. that focuses on advanced targeted online sales. https://innocanpharma.com/
For further information, please contact:
Iris Bincovich, CEO
+1-516-210-4025
+972-54-3012842
+442037699377
[email protected]
NEITHER THE CANADIAN SECURITIES EXCHANGE NOR ITS REGULATION SERVICES PROVIDER HAVE REVIEWED OR ACCEPT RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Cautionary note regarding forward-looking information
Certain information set forth in this news release, including, without limitation, information regarding research and development, collaborations, the filing of potential applications with the FDA and other regulatory authorities, the potential achievement of future regulatory milestones, the potential for treatment of conditions and other therapeutic effects resulting from research activities and/or the Company’s products, requisite regulatory approvals and the timing for market entry, is forward-looking information within the meaning of applicable securities laws. By its nature, forward-looking information is subject to numerous risks and uncertainties, some of which are beyond Innocan’s control. The forward-looking information contained in this news release is based on certain key expectations and assumptions made by Innocan, including expectations and assumptions concerning the anticipated benefits of the products, satisfaction of regulatory requirements in various jurisdictions and satisfactory completion of requisite production and distribution arrangements.
Forward-looking information is subject to various risks and uncertainties which could cause actual results and experience to differ materially from the anticipated results or expectations expressed in this news release. The key risks and uncertainties include but are not limited to: general global and local (national) economic, market and business conditions; governmental and regulatory requirements and actions by governmental authorities; and relationships with suppliers, manufacturers, customers, business partners and competitors. There are also risks that are inherent in the nature of product distribution, including import / export matters and the failure to obtain any required regulatory and other approvals (or to do so in a timely manner) and availability in each market of product inputs and finished products. The anticipated timeline for entry to markets may change for a number of reasons, including the inability to secure necessary regulatory requirements, or the need for additional time to conclude and/or satisfy the manufacturing and distribution arrangements. As a result of the foregoing, readers should not place undue reliance on the forward-looking information contained in this news release concerning the timing of launch of product distribution. A comprehensive discussion of other risks that impact Innocan can also be found in Innocan’s public reports and filings which are available under Innocan’s profile at www.sedarplus.ca.
Readers are cautioned that undue reliance should not be placed on forward-looking information as actual results may vary materially from the forward-looking information. Innocan does not undertake to update, correct or revise any forward-looking information as a result of any new information, future events or otherwise, except as may be required by applicable law.
Logo – https://mma.prnewswire.com/media/2046271/3968398/Innocan_Pharma_Corporation_Logo.jpg

![]() View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-reports-second-quarter-2024-results-with-revenue-growth-of-over-2-8x-to-8-6-million-302220337.html
View original content:https://www.prnewswire.co.uk/news-releases/innocan-pharma-reports-second-quarter-2024-results-with-revenue-growth-of-over-2-8x-to-8-6-million-302220337.html

-

 Cannabis2 weeks ago
Cannabis2 weeks agoSilly Nice Announces Launch of New 1G 510 Vape Cartridge and 2G All-In-One Vape in New York Dispensaries This September
-

 Cannabis2 weeks ago
Cannabis2 weeks agoCannabis Cultivation Market – Global Food Additives, Oils, Tinctures, Cannabis Indica, Cannabis Sativa Forecast 2024-2030
-

 Cannabis1 week ago
Cannabis1 week agoEurope Medical Cannabis Market Forecast 2024-2032: Tilray, Aurora Cannabis, and GW Pharmaceuticals Dominate the Market Landscape
-

 Cannabis2 weeks ago
Cannabis2 weeks agoGlobal Hemp Based Food Market Size To Worth USD 4.87 Billion By 2033 | CAGR Of 13.12%
-

 Innocan1 week ago
Innocan1 week agoInnocan Pharma Announces Closing of Private Placement and Grant of Stock Options
-
Indivior4 days ago
Indivior Provides Update on Aelis Farma’s Clinical Phase 2B Study Results with AEF0117 in Participants with Cannabis Use Disorder








